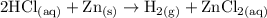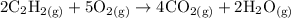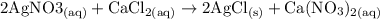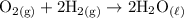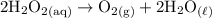Please help
Write a balanced equation for each chemical reactions. Include appropriate symbols. First reactant formula is given.
Hydrochloric acid (HCl) reacts with Zinc to form hydrogen gas and zinc chloride.
2 HCl +
Acetylene gas (C2H2) burns in a welding torch with oxygen to form carbon dioxide gas and water vapor. 2 C2H2 (g) +
c. Silver nitrate plus calcium chloride (CaCll2) yields silver chloride and calcium nitrate. 2 AgNO3 +
d. Oxygen gas combines with hydrogen gas to produce liquid water. O2 +
e. Hydrogen peroxide decomposes into oxygen gas and water when it contacts blood. 2 H2O2

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 18:30
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
Please help
Write a balanced equation for each chemical reactions. Include appropriate symbols. Fir...
Questions



Mathematics, 06.05.2020 21:34

History, 06.05.2020 21:34



English, 06.05.2020 21:34


Mathematics, 06.05.2020 21:34

English, 06.05.2020 21:34

Mathematics, 06.05.2020 21:34



Health, 06.05.2020 21:34


History, 06.05.2020 21:34

Biology, 06.05.2020 21:34