
Chemistry, 26.02.2021 03:20 makaylaf16571
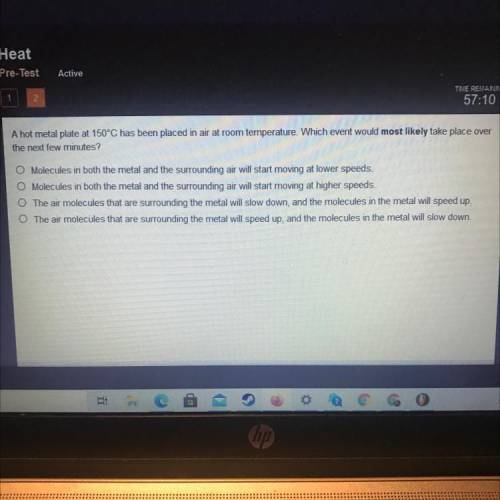
A hot metal plate at 150°C has been placed in air at room temperature. Which event would most likely take place over
the next few minutes?
A. Molecules in both the metal and the surrounding air will start moving at lower speeds.
B. Molecules in both the metal and the surrounding air will start moving at higher speeds.
C. The air molecules that are surrounding the metal will slow down, and the molecules in the metal will speed up.
D. The air molecules that are surrounding the metal will speed up, and the molecules in the metal will slow down

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 23.06.2019 04:40
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1
You know the right answer?
A hot metal plate at 150°C has been placed in air at room temperature. Which event would most likely...
Questions

Social Studies, 05.06.2020 04:02

Mathematics, 05.06.2020 04:02







Mathematics, 05.06.2020 04:02

Mathematics, 05.06.2020 04:02








Mathematics, 05.06.2020 04:02




