

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
If the molecules in the above illustration react to form NH3 according to the equation N2 3 H2 2 NH3...
Questions



English, 12.05.2021 18:20

Arts, 12.05.2021 18:20

Social Studies, 12.05.2021 18:20

Mathematics, 12.05.2021 18:20

Mathematics, 12.05.2021 18:20

Chemistry, 12.05.2021 18:20



Mathematics, 12.05.2021 18:20

Mathematics, 12.05.2021 18:20


Social Studies, 12.05.2021 18:20


Physics, 12.05.2021 18:20



Mathematics, 12.05.2021 18:20

Mathematics, 12.05.2021 18:20

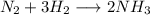
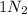 gives
gives 
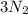 gives=
gives= 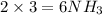
 gives
gives 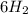 gives =
gives = 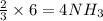
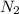
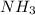 molecules were formed = 4.
molecules were formed = 4.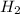 excess molecules are= 1
excess molecules are= 1

