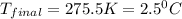Chemistry, 26.02.2021 06:20 princessss30188
A 100 mL sample of ethanol at 25°C is mixed with a 300 mL sample of ethanol at -5°C. The mixture is allowed to come to thermal equilibrium. What is the final temperature?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2
You know the right answer?
A 100 mL sample of ethanol at 25°C is mixed with a 300 mL sample of ethanol at -5°C. The mixture is...
Questions







History, 30.08.2019 01:40


Mathematics, 30.08.2019 01:40

Mathematics, 30.08.2019 01:40

Social Studies, 30.08.2019 01:40




Spanish, 30.08.2019 01:40

English, 30.08.2019 01:40

History, 30.08.2019 01:40

Business, 30.08.2019 01:40

Health, 30.08.2019 01:40

Mathematics, 30.08.2019 01:40

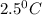
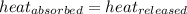
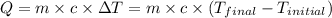
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/1150/2880/09236.png) .................(1)
.................(1)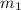 = mass of first sample of ethanol = 100 ml
= mass of first sample of ethanol = 100 ml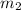 = mass of second sample of ethanol = 300 ml
= mass of second sample of ethanol = 300 ml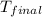 = final temperature = ?
= final temperature = ?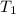 = temperature of first sample of ethanol =
= temperature of first sample of ethanol = 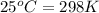
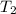 = temperature of second sample of ethanol =
= temperature of second sample of ethanol = 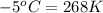
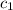 =
= 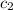 = specific heat of ethanol
= specific heat of ethanol![-100\times (T_{final}-298)=[300\times (T_{final}-268)]](/tpl/images/1150/2880/2eae3.png)