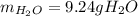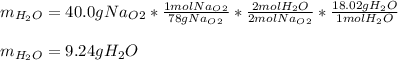Chemistry, 26.02.2021 09:20 milkshakegrande101
A) What mass in grams of H20 is needed to react completely with 40.0 g of
Na2O2?
M(H2O) =18.02g/mol
M(NA2O2)= 78g/mol
2Na2O2 (s)+2h2O(I)—> 4NaOH(aq) + O2 (g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
You know the right answer?
A) What mass in grams of H20 is needed to react completely with 40.0 g of
Na2O2?
M(H2O)...
M(H2O)...
Questions





Mathematics, 06.02.2021 01:00



History, 06.02.2021 01:00

Mathematics, 06.02.2021 01:00


Arts, 06.02.2021 01:00

Chemistry, 06.02.2021 01:00

English, 06.02.2021 01:00



History, 06.02.2021 01:00



Mathematics, 06.02.2021 01:00

Mathematics, 06.02.2021 01:00