
Chemistry, 27.02.2021 02:30 Havcscsgg4593
If there are 0.090 moles of sugar (C₁₂H₂₂O₁₁) in the average can of soda, how many sugar molecules would this be

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1


You know the right answer?
If there are 0.090 moles of sugar (C₁₂H₂₂O₁₁) in the average can of soda, how many sugar molecules w...
Questions

Mathematics, 18.03.2021 23:40







Mathematics, 18.03.2021 23:40



Chemistry, 18.03.2021 23:40



Computers and Technology, 18.03.2021 23:40



Mathematics, 18.03.2021 23:40

English, 18.03.2021 23:40

Mathematics, 18.03.2021 23:40

Mathematics, 18.03.2021 23:40

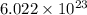 molecules of that compound.
molecules of that compound.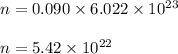
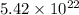 .
.

