
Chemistry, 28.02.2021 04:00 savthespice
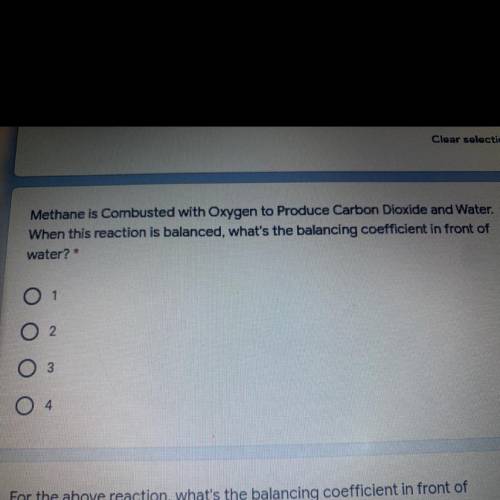
Methane is Combusted with Oxygen to Produce Carbon Dioxide and Water. 1 point
When this reaction is balanced, what's the balancing coefficient in front of
water? *
Op. 1
Op. 2
Op. 3
Op. 4

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1
You know the right answer?
Methane is Combusted with Oxygen to Produce Carbon Dioxide and Water. 1 point
When this reaction is...
Questions

Mathematics, 05.05.2020 05:56

Mathematics, 05.05.2020 05:56

Mathematics, 05.05.2020 05:56

Mathematics, 05.05.2020 05:56

History, 05.05.2020 05:56

Biology, 05.05.2020 05:56

Chemistry, 05.05.2020 05:56

English, 05.05.2020 05:56


Mathematics, 05.05.2020 05:56


History, 05.05.2020 05:56

Arts, 05.05.2020 05:56

Mathematics, 05.05.2020 05:56

History, 05.05.2020 05:56

Mathematics, 05.05.2020 05:56

Social Studies, 05.05.2020 05:56





