
Chemistry, 28.02.2021 06:40 twistedhyperboles
2c+02=2CO2. The moles of co2 produced when 0.25 moles of O2 react is?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
2c+02=2CO2. The moles of co2 produced when 0.25 moles of O2 react is?...
Questions

Health, 22.04.2021 21:50




English, 22.04.2021 21:50

Mathematics, 22.04.2021 21:50

Mathematics, 22.04.2021 21:50

Mathematics, 22.04.2021 21:50



Mathematics, 22.04.2021 21:50

Mathematics, 22.04.2021 21:50





Mathematics, 22.04.2021 21:50



Mathematics, 22.04.2021 21:50

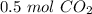
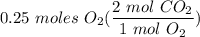 [DA] Multiply/Divide [Cancel out units]:
[DA] Multiply/Divide [Cancel out units]: 

