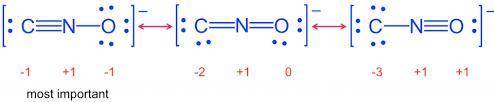Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3
You know the right answer?
Draw Lewis structures for the fulminate ion including possible resonance forms.
Draw the molecule b...
Questions

Mathematics, 27.06.2021 14:00


English, 27.06.2021 14:00

English, 27.06.2021 14:00

Social Studies, 27.06.2021 14:00

Mathematics, 27.06.2021 14:00


History, 27.06.2021 14:00

Biology, 27.06.2021 14:00


Mathematics, 27.06.2021 14:00



Mathematics, 27.06.2021 14:00

English, 27.06.2021 14:10

Mathematics, 27.06.2021 14:10

Mathematics, 27.06.2021 14:10

Social Studies, 27.06.2021 14:10

Mathematics, 27.06.2021 14:10

Social Studies, 27.06.2021 14:10