Chemistry, 28.02.2021 14:50 nataliemoore1974
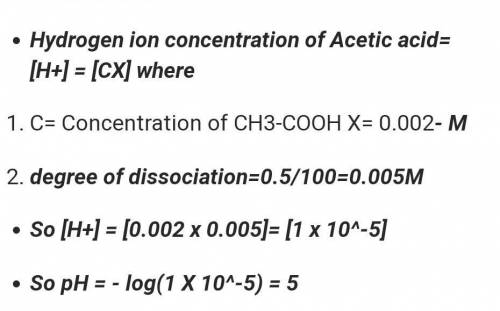
Calculate the pH of a 0.002 M acetic acid solution if it is 2.3% ionised at this dilution. Ka = 1.8 x 10-5.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
Calculate the pH of a 0.002 M acetic acid solution if it is 2.3% ionised at this dilution. Ka = 1.8...
Questions



Computers and Technology, 20.03.2020 10:09



Mathematics, 20.03.2020 10:09


Mathematics, 20.03.2020 10:09




Mathematics, 20.03.2020 10:09

Mathematics, 20.03.2020 10:09

Mathematics, 20.03.2020 10:09

Mathematics, 20.03.2020 10:09

Mathematics, 20.03.2020 10:09


Computers and Technology, 20.03.2020 10:10