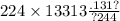Chemistry, 28.02.2021 18:50 ninaaforever
In the following take CV = 20.8 and CP = 29.1 J⋅mol−1⋅°C−1 for nitrogen gas: (a) Three moles of nitrogen at 30°C, contained in a rigid vessel, is heated to 250°C. How much heat is required if the vessel has a negligible heat capacity? If the vessel weighs 100 kg and has a heat capacity of 0.5 kJ⋅kg−1⋅°C−1, how much heat is required? (b) Four moles of nitrogen at 200°C is contained in a piston/cylinder arrangement. How much heat must be extracted from this system, which is kept at constant pressure, to cool it to 40°C if the heat capacity of the piston and cylinder is neglected?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1


Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
You know the right answer?
In the following take CV = 20.8 and CP = 29.1 J⋅mol−1⋅°C−1 for nitrogen gas: (a) Three moles of nitr...
Questions

Mathematics, 14.04.2021 18:40




Mathematics, 14.04.2021 18:40




History, 14.04.2021 18:40

Chemistry, 14.04.2021 18:40



Biology, 14.04.2021 18:40

Computers and Technology, 14.04.2021 18:40

Chemistry, 14.04.2021 18:40

Mathematics, 14.04.2021 18:40

Mathematics, 14.04.2021 18:40