
Chemistry, 28.02.2021 19:40 heavenwagner
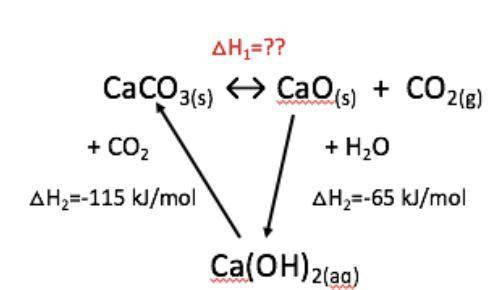
In this exercise you are asked to calculate the enthalpy change of lime burning, using the enthalpy values of the intermediary reactions. The Hess cycle diagram of the limestone cycle is enclosed for assistance.
CaCO3 ↔︎ CaO + CO2 △H1 = ???
CaO + H2O = Ca(OH)2 △H2 = -65 kJ/mol
Ca(OH)2 + CO2 = CaCO3 + H2O △H3 = -115 kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1
You know the right answer?
In this exercise you are asked to calculate the enthalpy change of lime burning, using the enthalpy...
Questions

Biology, 03.10.2019 13:50

History, 03.10.2019 13:50



Health, 03.10.2019 13:50

Mathematics, 03.10.2019 13:50

Mathematics, 03.10.2019 13:50



Biology, 03.10.2019 13:50

History, 03.10.2019 13:50

Mathematics, 03.10.2019 13:50


English, 03.10.2019 13:50



Mathematics, 03.10.2019 13:50

History, 03.10.2019 13:50





