
Chemistry, 28.02.2021 21:10 cicimarie2018
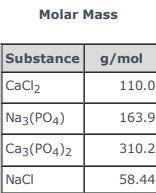
3CaCl2(aq) + 2Na3(PO4)(aq) → Ca3(PO4)2(s)+ 6NaCl(aq) Use the balanced equation and the Molar Mass table above to answer the following question. Suppose 163.9 g of Na3(PO4) in solution mixed with sufficient CaCl2 in solution yields 116 g ofCa3(PO4)2(s). What is the percent yield of Ca3(PO4)2(s)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
The density of a planet is 0.69 g/cm3 (density of water is 1.0 g/cm3). which of the following planets might this be? a. mercury b. venus c. saturn d. mars
Answers: 3

Chemistry, 21.06.2019 17:30
Which type of stress results when two plates push against one another? a. compression b. tension c. force d. shear
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
You know the right answer?
3CaCl2(aq) + 2Na3(PO4)(aq) → Ca3(PO4)2(s)+ 6NaCl(aq) Use the balanced equation and the Molar Mass ta...
Questions

English, 11.07.2019 12:50

Biology, 11.07.2019 12:50



History, 11.07.2019 12:50

Physics, 11.07.2019 12:50


Mathematics, 11.07.2019 12:50

Chemistry, 11.07.2019 12:50


Mathematics, 11.07.2019 12:50



Mathematics, 11.07.2019 12:50


Chemistry, 11.07.2019 12:50

Mathematics, 11.07.2019 12:50


Mathematics, 11.07.2019 12:50

Chemistry, 11.07.2019 12:50



