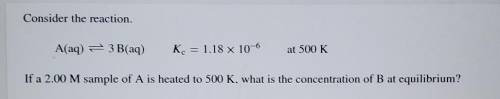Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
Consider the reaction. A(aq) = 3 B(aq) K. = 1.18 x 10^-6 at 500K If a 2.00 M sample of A is heated t...
Questions



Chemistry, 29.09.2019 07:30


Advanced Placement (AP), 29.09.2019 07:30

Biology, 29.09.2019 07:30


History, 29.09.2019 07:30


Mathematics, 29.09.2019 07:30


English, 29.09.2019 07:30



Computers and Technology, 29.09.2019 07:30



Mathematics, 29.09.2019 07:30