
Chemistry, 01.03.2021 04:10 krystalhurst97
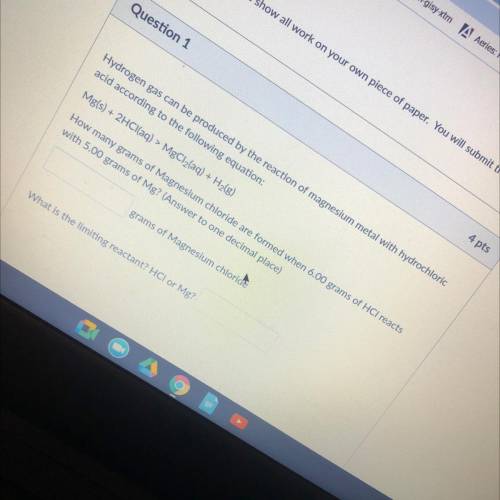
Hydrogen gas can be produced by the reaction of magnesium metal with hydrochloric
acid according to the following equation:
Mg(s) + 2HCl(aq) > MgCl2(aq) + H2(g)
How many grams of Magnesium chloride are formed when 6.00 grams of HCl reacts
with 5.00 grams of Mg? (Answer to one decimal place)
grams of Magnesium chloride
What is the limiting reactant? HCI or Mg?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 04:00
Actual ingredients of lab (the cookies i am actually making) 1/2 cup sugar 1/2 cup brown sugar 1 1/3 stick margarine 1 egg 1/2 tsp salt 1 tsp vanilla 1/2 tsp baking soda 1 1/2 cup flour 1 1/3 cup chocolate chip can you answer the questions below ? discussion 1. suppose you are given the following amounts of ingredients: 1 dozen eggs 24 tsp. of vanilla 1 lb. (82 tsp.) of salt 1 lb. (84 tsp.) of baking soda 3 cups of chocolate chips 5 lb. (11 cups) of sugar 2 lb. (4 cups) of brown sugar 1 lb. (4 sticks) of margarine a. for each ingredient, calculate how many cookies could be prepared if all of that ingredient were consumed. (for example, the recipe shows that using 1 egg- with the right amounts of the other ingredients- yields 24 cookies. how many cookies can you make if the recipe is increased proportionately for 12 eggs? ) b. to determine the limiting reactant for the new ingredients list, identify which ingredient will result in the fewest number of cookies. c. what is the maximum number of cookies that can be produced from the new amounts of ingredients?
Answers: 1

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
Hydrogen gas can be produced by the reaction of magnesium metal with hydrochloric
acid according to...
Questions


Mathematics, 24.11.2020 23:10


English, 24.11.2020 23:10


Biology, 24.11.2020 23:10


Mathematics, 24.11.2020 23:10


Mathematics, 24.11.2020 23:10



Mathematics, 24.11.2020 23:10

Biology, 24.11.2020 23:10

Mathematics, 24.11.2020 23:10

History, 24.11.2020 23:10

Arts, 24.11.2020 23:10


Mathematics, 24.11.2020 23:10



