
Chemistry, 01.03.2021 04:50 mraymundo025p0gpw9
A 2.00mol sample of C2H5OH undergoes the phase transition illustrated in the diagram above. The molar enthalpy of vaporization, ΔHvap, of C2H5OH is +38.6kJ/mol. Which of the following best identifies the change in enthalpy in the phase transition shown in the diagram?
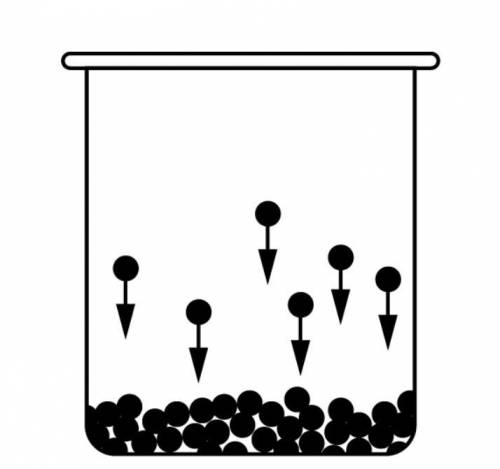

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1
You know the right answer?
A 2.00mol sample of C2H5OH undergoes the phase transition illustrated in the diagram above. The mola...
Questions

Social Studies, 21.04.2021 04:00

Mathematics, 21.04.2021 04:00



Mathematics, 21.04.2021 04:00



Mathematics, 21.04.2021 04:00

Mathematics, 21.04.2021 04:00

Mathematics, 21.04.2021 04:00


Mathematics, 21.04.2021 04:00


Arts, 21.04.2021 04:00

History, 21.04.2021 04:00

Mathematics, 21.04.2021 04:00




Mathematics, 21.04.2021 04:00



