
Chemistry, 01.03.2021 08:20 Bradgarner772
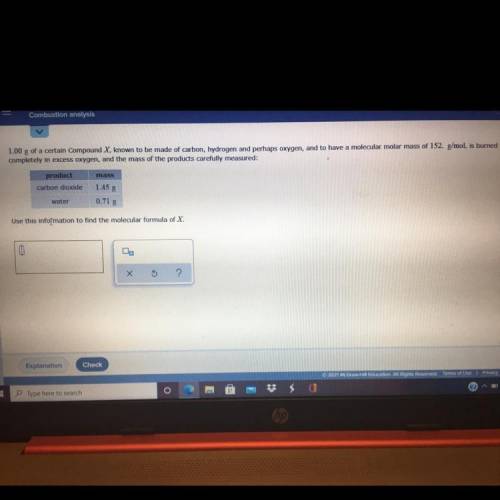
1.00 g of a certain Compound X known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 152. g/mol, is burned
completely in excess oxygen, and the mass of the products carefully measured:
mass
product
carbon dioxide
1.458
0.718
water
use this information to find the molecular formula of X.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1


Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
1.00 g of a certain Compound X known to be made of carbon, hydrogen and perhaps oxygen, and to have...
Questions

Mathematics, 28.07.2019 04:33




Mathematics, 28.07.2019 04:33



English, 28.07.2019 04:33

History, 28.07.2019 04:33

English, 28.07.2019 04:33



Mathematics, 28.07.2019 04:33

Mathematics, 28.07.2019 04:33



English, 28.07.2019 04:33

Social Studies, 28.07.2019 04:33

Mathematics, 28.07.2019 04:33

Mathematics, 28.07.2019 04:33



