=
Breanna
Combustion analysis
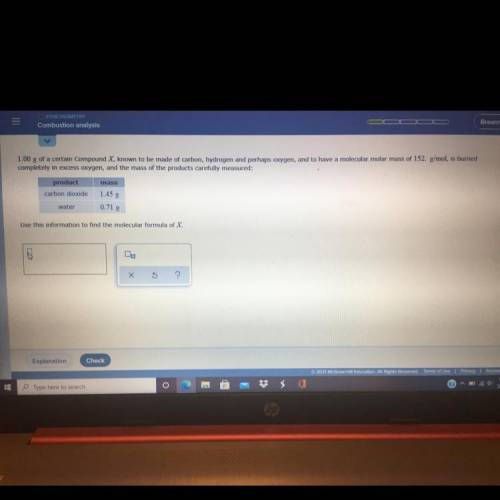
1.00 g of a certain Compound X, known to be made of carbo...

=
Breanna
Combustion analysis
1.00 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 152. g/mol, is burned
completely in excess oxygen, and the mass of the products carefully measured:
mass
product
carbon dioxide
1.45 g
0.71 g
water
do
Use this information to find the molecular formula of X
B
Х
5
?
Explanation
Check
2021 McGraw He Education. All Rights Reserved. Terms of Use Privacy | Access
11:35 PM
() Ap司
2/28/2021
O
Type here to search

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1
You know the right answer?
Questions


Mathematics, 28.05.2020 04:00



Mathematics, 28.05.2020 04:00



History, 28.05.2020 04:00



Mathematics, 28.05.2020 04:00

History, 28.05.2020 04:00







English, 28.05.2020 04:00




