Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2
You know the right answer?
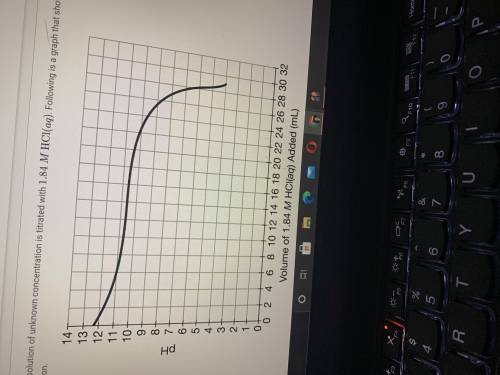
If 28.25mL of 1.84MHCl(aq) was required to reach the equivalence point, calculate the concentration...
Questions

Mathematics, 18.09.2019 06:30






History, 18.09.2019 06:30



Mathematics, 18.09.2019 06:30



History, 18.09.2019 06:30

Chemistry, 18.09.2019 06:30




Mathematics, 18.09.2019 06:30


Biology, 18.09.2019 06:30