
Chemistry, 01.03.2021 18:30 Matseleng3775
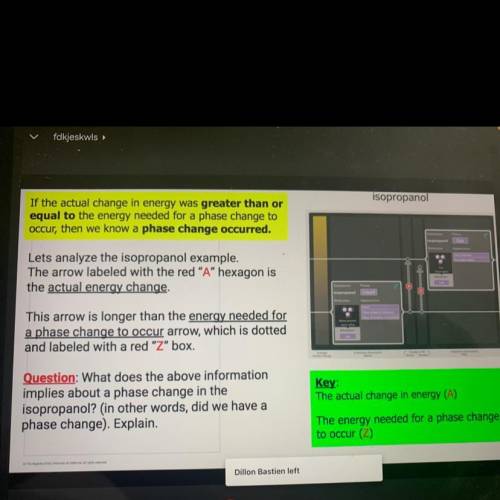
If the actual change in energy was greater than o
equal to the energy needed for a phase change to
occur, then we know a phase change occurred.
Lets analyze the isopropanol example,
The arrow labeled with the red "A" hexagon is
the actual energy change.
This arrow is longer than the energy needed for
a phase change to occur arrow, which is dotted
and labeled with a red "Z" box.
Question: What does the above information
implies about a phase change in the
isopropanol? (in other words, did we have a
phase change). Explain.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 23.06.2019 00:00
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
You know the right answer?
If the actual change in energy was greater than o
equal to the energy needed for a phase change to<...
Questions


Arts, 12.05.2021 16:40






Mathematics, 12.05.2021 16:40

Mathematics, 12.05.2021 16:40

Mathematics, 12.05.2021 16:40

Mathematics, 12.05.2021 16:40


Spanish, 12.05.2021 16:40


Mathematics, 12.05.2021 16:40

Mathematics, 12.05.2021 16:40


Mathematics, 12.05.2021 16:40

Mathematics, 12.05.2021 16:40



