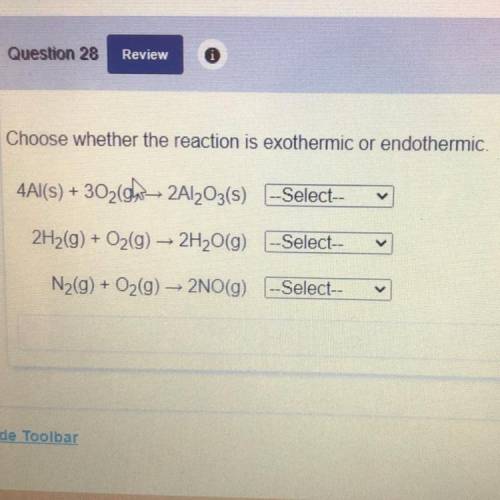
Choose whether the reaction is exothermic or endothermic.
4Al(s) + 3020w 2Al2O3(s) --Select--
...

Chemistry, 01.03.2021 20:10 ilovecatsomuchlolol
Choose whether the reaction is exothermic or endothermic.
4Al(s) + 3020w 2Al2O3(s) --Select--
V
2H2(g) + O2(g) → 2H2O(9) --Select--
N2(g) + O2(g) - 2NO(g) -Select--

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
Questions


Mathematics, 21.01.2021 21:40

Mathematics, 21.01.2021 21:40

History, 21.01.2021 21:40






Mathematics, 21.01.2021 21:40

Mathematics, 21.01.2021 21:40

Health, 21.01.2021 21:40



Spanish, 21.01.2021 21:40

Mathematics, 21.01.2021 21:40




Mathematics, 21.01.2021 21:40



