
Chemistry, 01.03.2021 21:50 lanaiheart7
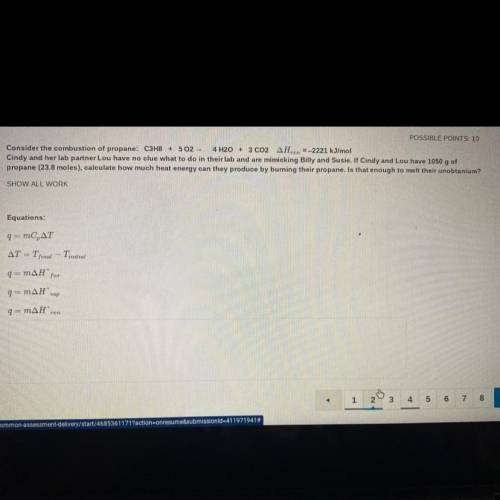
Consider the combustion of propane: C3H8 + 502 - 4 H2O + 3 CO2 AHzn =-2221 kJ/mol
Cindy and her lab partner Lou have no clue what to do in their lab and are mimicking Billy and Susie. If Cindy and Lou have 1050 g of
propane (23.8 moles), calculate how much heat energy can they produce by burning their propane. Is that enough to melt their unobtanium?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

You know the right answer?
Consider the combustion of propane: C3H8 + 502 - 4 H2O + 3 CO2 AHzn =-2221 kJ/mol
Cindy and her lab...
Questions


Mathematics, 13.10.2020 02:01


Social Studies, 13.10.2020 02:01


German, 13.10.2020 02:01

Geography, 13.10.2020 02:01

History, 13.10.2020 02:01



English, 13.10.2020 02:01







Physics, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01

Business, 13.10.2020 02:01



