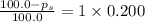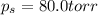Chemistry, 01.03.2021 22:00 lisilyn9755
What is the vapor pressure of a solution in which the mole fraction of the solute is 0.200 and the vapor pressure of the pure solvent is 100.0 torr? (Assume a single nonvolatile, nonelectrolyte solute).
a. 0 torr
b. 80.0 torr
c. 100.0 torr
d. 120.0 torr
e. 20.0 torr

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
You know the right answer?
What is the vapor pressure of a solution in which the mole fraction of the solute is 0.200 and the v...
Questions

Mathematics, 17.11.2020 01:50

History, 17.11.2020 01:50

English, 17.11.2020 01:50


English, 17.11.2020 01:50

Chemistry, 17.11.2020 01:50


History, 17.11.2020 01:50


Mathematics, 17.11.2020 01:50


History, 17.11.2020 01:50

Arts, 17.11.2020 01:50

Mathematics, 17.11.2020 01:50

Business, 17.11.2020 01:50

English, 17.11.2020 01:50

English, 17.11.2020 01:50


Mathematics, 17.11.2020 01:50

Mathematics, 17.11.2020 01:50

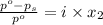
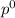 = vapor pressure of pure solvent = 100.0 torr
= vapor pressure of pure solvent = 100.0 torr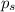 = vapor pressure of solution = ?
= vapor pressure of solution = ?
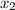 = mole fraction of solute = 0.200
= mole fraction of solute = 0.200