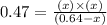Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2


Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3
You know the right answer?
If 0.64 mol PCl5 is placed in a 1.0 L flask and allowed to reach equilibrium at a given temperature,...
Questions





Social Studies, 22.10.2020 20:01

Mathematics, 22.10.2020 20:01


Mathematics, 22.10.2020 20:01

Mathematics, 22.10.2020 20:01

Mathematics, 22.10.2020 20:01



Biology, 22.10.2020 20:01



Business, 22.10.2020 20:01


History, 22.10.2020 20:01

English, 22.10.2020 20:01

History, 22.10.2020 20:01

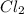 at equilibrium is 0.36 M
at equilibrium is 0.36 M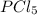 = 0.64 mole
= 0.64 mole 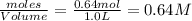
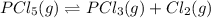
![K_c=\frac{[Cl_2]\times [PCl_3]}{[PCl_5]}](/tpl/images/1158/2042/ffe89.png)