Chemistry, 01.03.2021 22:50 thebrain1345
Please answer with an actual answer and not just put a random word ^-^
Materials
large ball about the size of a basketball
small ball about the size of a tennis ball
strong light of about 100 watts or more
dark room
Directions
Place the large ball about 12 feet from the light source. Then place the small ball in the shadow of the large ball. Observe the shadow pattern.
Place the large ball in the shadow of the small ball. Observe the shadow pattern.
Questions
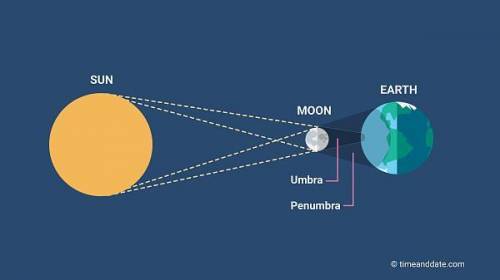
Which eclipse was modeled when the large ball was between the small ball and the light?
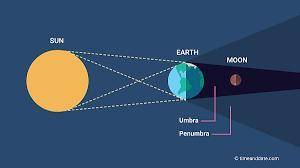
Which eclipse was modeled when the small ball was between the large ball and the light?
What does the large ball represent?
What does the small ball represent?
What does the light source represent?
Draw a picture showing a lunar eclipse.
Draw a picture showing a solar eclipse.
Upload your answers to the experiment questions and your drawings of a lunar and solar eclipse. Be sure to answer every question and use complete sentences.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
Please answer with an actual answer and not just put a random word ^-^
Materials
large...
large...
Questions

Mathematics, 13.05.2021 19:20


Mathematics, 13.05.2021 19:20



Mathematics, 13.05.2021 19:20

Mathematics, 13.05.2021 19:20

English, 13.05.2021 19:20

Computers and Technology, 13.05.2021 19:20


Mathematics, 13.05.2021 19:20

Mathematics, 13.05.2021 19:20


Mathematics, 13.05.2021 19:20

Mathematics, 13.05.2021 19:20





Mathematics, 13.05.2021 19:20