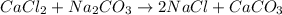Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
Calculate the volume in mL of 0.100 of CaCl2 needed to produce 1.00 g of CaCO3. there is an excess o...
Questions

Social Studies, 03.02.2020 17:44

English, 03.02.2020 17:44




Social Studies, 03.02.2020 17:44

English, 03.02.2020 17:44


English, 03.02.2020 17:44

Arts, 03.02.2020 17:44

Mathematics, 03.02.2020 17:44



Mathematics, 03.02.2020 17:44


Advanced Placement (AP), 03.02.2020 17:44



Mathematics, 03.02.2020 17:44