Chemistry, 02.03.2021 03:40 makailaaa2
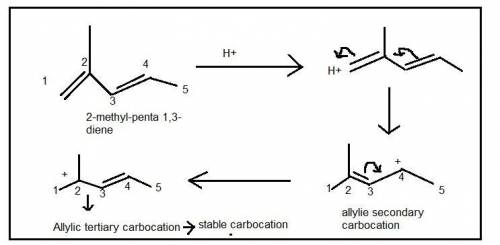
5. Write the two resonance hybrids for the carbocation that would be formed by protonation at C-1 of 2-methyl-1,3-pentadiene. Without doing a calculation, would you expect C-2 or C-4 (the two end carbons of the allylic cation) to have the most positive charge on it

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1
You know the right answer?
5. Write the two resonance hybrids for the carbocation that would be formed by protonation at C-1 of...
Questions

Computers and Technology, 08.07.2019 23:00

Mathematics, 08.07.2019 23:00


Mathematics, 08.07.2019 23:00







English, 08.07.2019 23:00

Business, 08.07.2019 23:00


Computers and Technology, 08.07.2019 23:00

History, 08.07.2019 23:00




Mathematics, 08.07.2019 23:00