
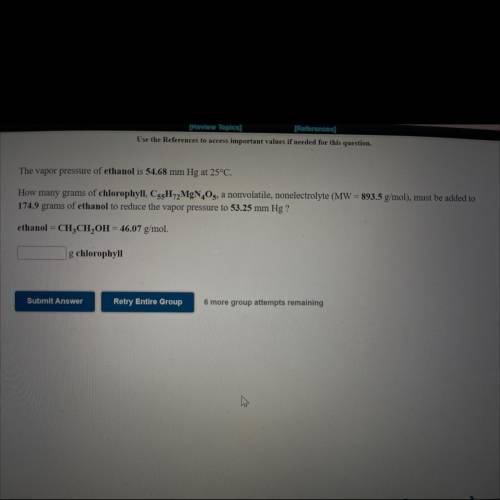
The vapor pressure of ethanol is 54.68 mm Hg at 25°C.
How many grams of chlorophyll, C55H72MgN4O5, a nonvolatile, nonelectrolyte (MW = 893.5 g/mol), must be added to
174.9 grams of ethanol to reduce the vapor pressure to 53.25 mm Hg ?
ethanol = CH3CH2OH = 46.07 g/mol.
How many ___g chlorophyll?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3
You know the right answer?
The vapor pressure of ethanol is 54.68 mm Hg at 25°C.
How many grams of chlorophyll, C55H72MgN4O5,...
Questions


History, 06.01.2021 23:00


Mathematics, 06.01.2021 23:00

Health, 06.01.2021 23:00

Mathematics, 06.01.2021 23:00


Mathematics, 06.01.2021 23:00

English, 06.01.2021 23:00


Mathematics, 06.01.2021 23:00

Mathematics, 06.01.2021 23:00

Mathematics, 06.01.2021 23:00

Chemistry, 06.01.2021 23:00





Medicine, 06.01.2021 23:00

Mathematics, 06.01.2021 23:00



