
Chemistry, 02.03.2021 08:40 nataliem02
PLEASE HELP PLEASE HELP PLEASE HELP PLEASE HELP PLEASE HELP PLEASE HELP PLEASE HELP PLEASE HELP PLEASE HELP PLEWSE HELP PLEASE HELP ANYBODY DIMENSIONAL ANALYSIS
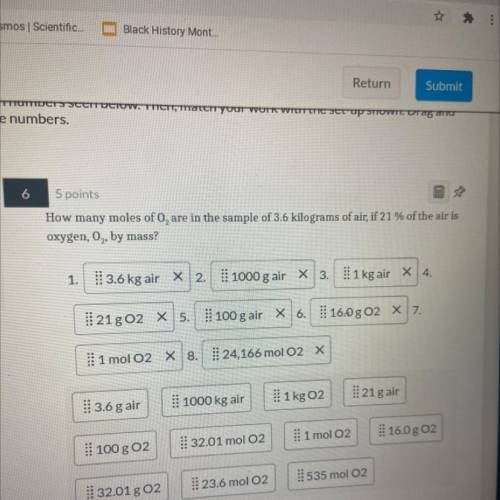
How many moles of O2 are in the sample of 3.6 kilograms of air if 21% of the air is oxygen, O2, by mass?
WILL GIVE BRAINLIEST PLEASE ANYONE WHO KNOWS HOW TO DO MOLE CONVERSIONS AND DIMENSIONAL ANALYSIS

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 23.06.2019 03:30
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1

Chemistry, 23.06.2019 06:30
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3

Chemistry, 23.06.2019 08:30
According to the passage, which of these is true about gray water systems? a) gray water systems use plants that require less water. eliminate b) gray water systems require the use of less fossil fuels. c) gray water systems reduce the amount of fresh water used. d) gray water systems reduce the amount water used by shower heads.
Answers: 1
You know the right answer?
PLEASE HELP PLEASE HELP PLEASE HELP PLEASE HELP PLEASE HELP PLEASE HELP PLEASE HELP PLEASE HELP PLEA...
Questions

Mathematics, 09.10.2020 22:01


Social Studies, 09.10.2020 22:01




History, 09.10.2020 22:01

English, 09.10.2020 22:01







Mathematics, 09.10.2020 22:01

Mathematics, 09.10.2020 22:01

Biology, 09.10.2020 22:01



Mathematics, 09.10.2020 22:01



