
Chemistry, 02.03.2021 14:00 mathman783
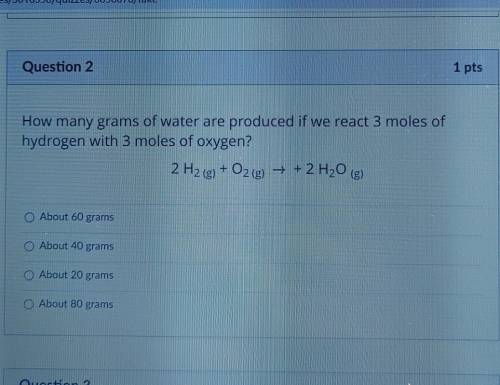
How many grams of water are produced if we react 3 moles of hydrogen with 3 moles of oxygen?
About 60 grams
About 40 grams
About 20 grams
About 80 grams

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
How many grams of water are produced if we react 3 moles of hydrogen with 3 moles of oxygen?
About...
Questions


Mathematics, 04.05.2021 15:50





Mathematics, 04.05.2021 15:50

English, 04.05.2021 15:50



Business, 04.05.2021 15:50


Computers and Technology, 04.05.2021 15:50



Mathematics, 04.05.2021 15:50

English, 04.05.2021 15:50


Biology, 04.05.2021 15:50

English, 04.05.2021 15:50



