
Chemistry, 02.03.2021 14:00 camiserjai1832
Ok we trying this again. so just in case if the picture isnt showing, here are the questions:
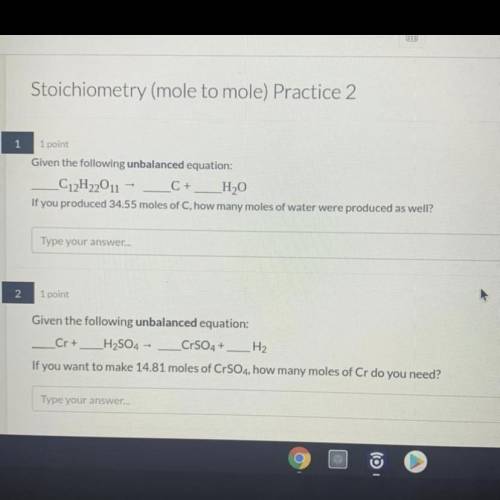
1. Given the unbalanced equation:
C12H22O11 = C + H2O
If you produced 34.55 moles of C, how many moles of water were produced as well?
2. Given the unbalanced equation:
Cr + H2SO4 = CrSO4 + H2
If you want to make 14.81 moles of CrSO4, how many moles of Cr do you need?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 23.06.2019 13:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
Ok we trying this again. so just in case if the picture isnt showing, here are the questions:
1. Gi...
Questions

Mathematics, 06.11.2021 05:20






Mathematics, 06.11.2021 05:20


Mathematics, 06.11.2021 05:20


Mathematics, 06.11.2021 05:20

Mathematics, 06.11.2021 05:20







History, 06.11.2021 05:20



