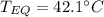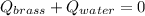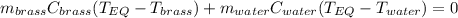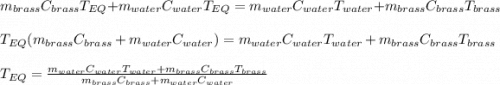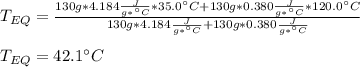Chemistry, 02.03.2021 14:00 LarryJoeseph
A 130 g sample of brass at 120.0 degrees Celsius is placed in a calorimeter cup that contains
130 g of water at 35.0 degrees Celsius. Disregard the absorption of heat by the cup and
calculate the final temperature of the brass and water. Specific heat of water = 4.18 J/gC,
specific heat of brass=0.380 J/gC. Attach your complete solution

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1


Chemistry, 23.06.2019 03:00
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers: 3
You know the right answer?
A 130 g sample of brass at 120.0 degrees Celsius is placed in a calorimeter cup that contains
130 g...
Questions

English, 14.12.2020 03:10

Biology, 14.12.2020 03:10

Mathematics, 14.12.2020 03:10

Advanced Placement (AP), 14.12.2020 03:10


History, 14.12.2020 03:10



Mathematics, 14.12.2020 03:10


Mathematics, 14.12.2020 03:10


Mathematics, 14.12.2020 03:10


History, 14.12.2020 03:10





Biology, 14.12.2020 03:10