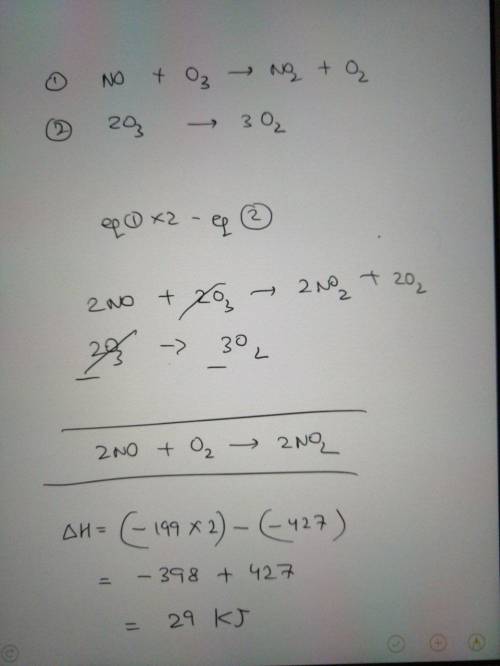Nitric oxide gas, NO(g), can be oxidized in air to
produce nitrogen dioxide gas, NO2(g):
2 NO...

Chemistry, 02.03.2021 14:40 dinosaur10
Nitric oxide gas, NO(g), can be oxidized in air to
produce nitrogen dioxide gas, NO2(g):
2 NO(g) + O2(g) → 2 NO2(g)
Determine the enthalpy change for this reaction
using any of these thermochemical equations:
02(g) →20(g)
AH = +495 kJ

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
Questions

Mathematics, 29.06.2021 01:00




Mathematics, 29.06.2021 01:00

Mathematics, 29.06.2021 01:00



English, 29.06.2021 01:00



Biology, 29.06.2021 01:00



Mathematics, 29.06.2021 01:00

Mathematics, 29.06.2021 01:00


Mathematics, 29.06.2021 01:00