Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
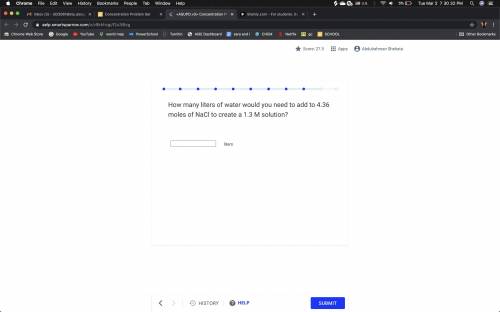
HELP PLZ How many liters of water would you need to add to 4.36 moles of NaCl to create a 1.3 M solu...
Questions


Mathematics, 28.08.2020 02:01



Mathematics, 28.08.2020 02:01


Mathematics, 28.08.2020 02:01






English, 28.08.2020 02:01


History, 28.08.2020 02:01

Mathematics, 28.08.2020 02:01


Engineering, 28.08.2020 02:01

Mathematics, 28.08.2020 02:01