
Chemistry, 02.03.2021 22:00 carolinehodges
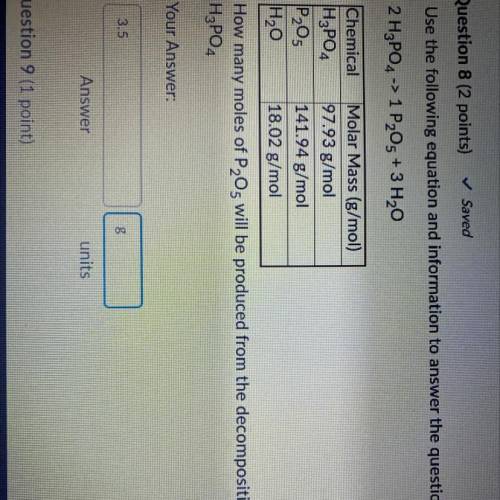
Use the following equation and information to answer the question that follows
2 H3PO4 -> 1 P205 + 3 H20
Chemical
Molar Mass (g/mol)
| H₂PO4 97.93 g/mol
P₂O5 141.94 g/mol
H2O 18.02 g/mol
How many moles of P2O5 will be produced from the decomposition of 0.833 mol of
H3PO4

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1


You know the right answer?
Use the following equation and information to answer the question that follows
2 H3PO4 -> 1 P205...
Questions


English, 04.10.2019 18:30

English, 04.10.2019 18:30


Mathematics, 04.10.2019 18:30

Mathematics, 04.10.2019 18:30







Mathematics, 04.10.2019 18:30



English, 04.10.2019 18:30


Mathematics, 04.10.2019 18:30




