
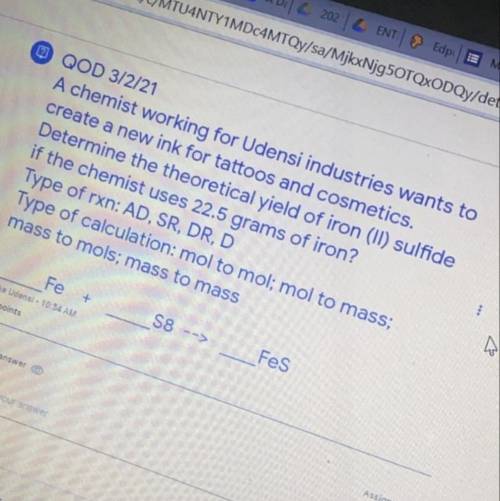
A chemist working for Udensi industries wants to create a new ink for tattoos and cosmetics. Determine the theoretical yield of iron (ll) sulfide if the chemist uses 22.5 grams of iron ? Type of rxn : AD , SR, DR , D Type of calculation : mol to mol ; mol to mass ; mass to mols mass to mass Fe S8--> Fes

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is a character of nuclear fusion but not nuclear fission
Answers: 3

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2
You know the right answer?
A chemist working for Udensi industries wants to create a new ink for tattoos and cosmetics. Determi...
Questions

Health, 07.01.2022 20:40

Mathematics, 07.01.2022 20:40

Mathematics, 07.01.2022 20:40

Social Studies, 07.01.2022 20:40

Mathematics, 07.01.2022 20:40


History, 07.01.2022 20:50


History, 07.01.2022 20:50



Chemistry, 07.01.2022 20:50




Advanced Placement (AP), 07.01.2022 20:50

SAT, 07.01.2022 20:50



Social Studies, 07.01.2022 20:50



