Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
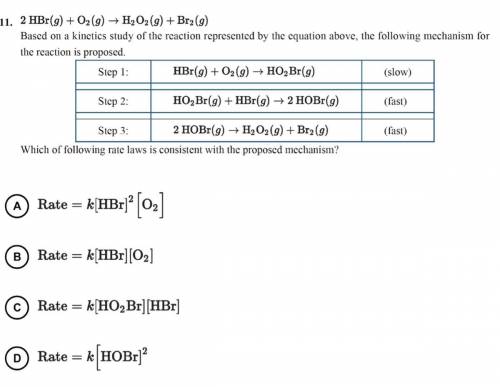
2 HBr(g)+O2(g)—>H2O2(g)+Br2(g)
Based on a kinetics study of the reaction represented by the equ...
Questions


Mathematics, 10.10.2019 19:00


Biology, 10.10.2019 19:00

Mathematics, 10.10.2019 19:00








English, 10.10.2019 19:00