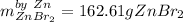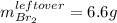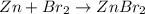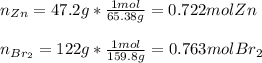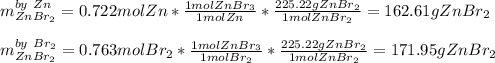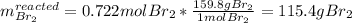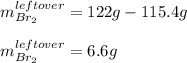Chemistry, 03.03.2021 08:40 parkermacyow71bm
A. Which reactant is the limiting reagent?
b. How much product is formed? (calculate for both if 2)
c. How much of the excess reagent remains?
1) 47.2 g of zinc metal react with 122 g of bromine in a closed container,
Final answers
la.
1b.
1c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2
You know the right answer?
A. Which reactant is the limiting reagent?
b. How much product is formed? (calculate for both if 2)...
Questions

Health, 29.07.2019 22:00

Mathematics, 29.07.2019 22:00

History, 29.07.2019 22:00


English, 29.07.2019 22:00






History, 29.07.2019 22:00


Computers and Technology, 29.07.2019 22:00


Biology, 29.07.2019 22:00


Mathematics, 29.07.2019 22:00


Social Studies, 29.07.2019 22:00