
A buffer solution contains 0.345 M acetic acid and 0.377 M sodium acetate . If 0.0613 moles of potassium hydroxide are added to 250 mL of this buffer, what is the pH of the resulting solution ? (Assume that the volume does not change upon adding potassium hydroxide. )

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3


Chemistry, 23.06.2019 09:30
How many moles of na2s2o3 are needed to react with 0.12mol of cl2? show work.
Answers: 1
You know the right answer?
A buffer solution contains 0.345 M acetic acid and 0.377 M sodium acetate . If 0.0613 moles of potas...
Questions



Mathematics, 19.11.2020 18:40


English, 19.11.2020 18:40




Mathematics, 19.11.2020 18:40


Mathematics, 19.11.2020 18:40





Mathematics, 19.11.2020 18:40

Biology, 19.11.2020 18:40



Mathematics, 19.11.2020 18:40

![\frac{[CH_3COO^-]}{[CH_3COOH]}](/tpl/images/1163/7311/4fc64.png)
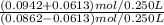 = 5.54
= 5.54

