
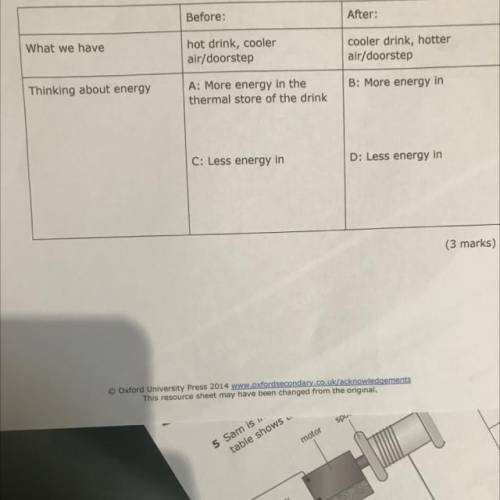
You place a hot drink outside on the doorstep on a cold day.
b Think about what happens in terms of energy, and fill in parts B, C, and D in the table
below. Part A has been done for you.
Before:
After:
What we have
hot drink, cooler
air/doorstep
cooler drink, hotter
air/doorstep
Thinking about energy
A: More energy in the
thermal store of the drink
B: More energy in
C: Less energy in
D: Less energy in

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 11:00
Problem page combustion of hydrocarbons such as pentane ( c5 h12 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.(a) write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. (b) suppose 0.350 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 degree c. calculate the volume of carbon dioxide gas that is produced.be sure your answer has the correct number of significant digits.
Answers: 2

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 23.06.2019 12:30
The equilibrium constant kc for the reaction 2 nocl(g) → 2 no(g) + cl2(g) is 0.453 at a certain temperature. a mixture of nocl, no, and cl2 with concentrations 1.30, 1.20, and 0.600 m, respectively, was introduced into a container at this temperature. which of the following is true? 1. no apparent reaction takes place. 2. [cl2] = 0.30 m at equilibrium. 3. nocl(g) is produced until equilibrium is reached. 4. [nocl] = [no] = [cl2] at equilibrium. 5. cl2(g) is produced until equilibrium is
Answers: 3
You know the right answer?
You place a hot drink outside on the doorstep on a cold day.
b Think about what happens in terms of...
Questions










Chemistry, 02.10.2019 06:00

History, 02.10.2019 06:00

English, 02.10.2019 06:00

Biology, 02.10.2019 06:00

English, 02.10.2019 06:00


Mathematics, 02.10.2019 06:00

Mathematics, 02.10.2019 06:00





