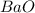Chemistry, 03.03.2021 18:40 jforeman42
What is the formula for the stable binary ionic compound formed between Barium and Oxygen?
A BaO
B. Ba2O2
CBaO2
D. Ba2O

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1
You know the right answer?
What is the formula for the stable binary ionic compound formed between Barium and Oxygen?
A BaO
Questions




English, 09.11.2020 19:20

Mathematics, 09.11.2020 19:20

Chemistry, 09.11.2020 19:20

Mathematics, 09.11.2020 19:20

Mathematics, 09.11.2020 19:20

English, 09.11.2020 19:20





Geography, 09.11.2020 19:20

Mathematics, 09.11.2020 19:20

Mathematics, 09.11.2020 19:20


Biology, 09.11.2020 19:20


Mathematics, 09.11.2020 19:20

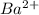 cation and
cation and 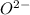 is an anion with oxidation state of -2. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral
is an anion with oxidation state of -2. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral