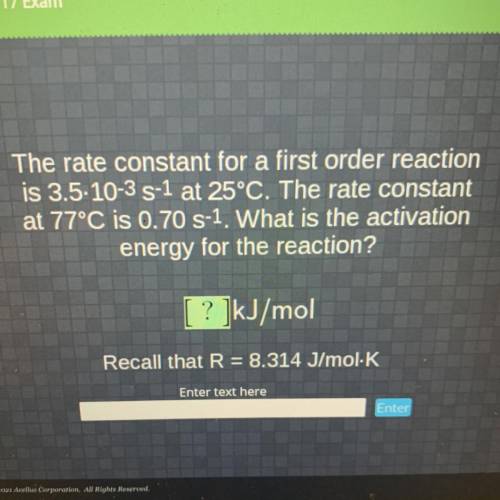
The rate constant for a first order reaction
is 3.5-10-3 S-1 at 25°C. The rate constant
at 77...

Chemistry, 03.03.2021 22:00 ryanzl1291
The rate constant for a first order reaction
is 3.5-10-3 S-1 at 25°C. The rate constant
at 77°C is 0.70 S-1. What is the activation
energy for the reaction?
[ ? ]kJ/mol
Recall that R = 8.314 J/mol K
-hurry it’s a test

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1
You know the right answer?
Questions

Mathematics, 07.01.2021 17:10

Chemistry, 07.01.2021 17:10

Mathematics, 07.01.2021 17:10

English, 07.01.2021 17:10

Mathematics, 07.01.2021 17:10



Mathematics, 07.01.2021 17:10


Mathematics, 07.01.2021 17:10

Mathematics, 07.01.2021 17:10



English, 07.01.2021 17:10



Physics, 07.01.2021 17:10





