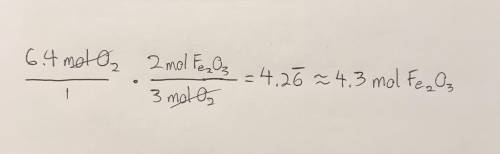Chemistry, 04.03.2021 01:10 lokaranjan5736
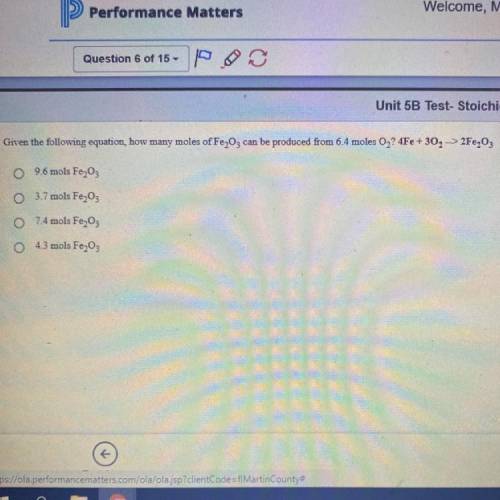
Given the following equation, how many moles of Fe2O3 can be produced from 6.4 moles O2? 4Fe +3O2 - - > 2Fe2O3

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Pbco3 –> pbo+ co2. how many liters of carbon dioxide gas is produced from the decomposition of 32 grams of lead (ll) carbonate?
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
Given the following equation, how many moles of Fe2O3 can be produced from 6.4 moles O2?
4Fe +3O2 -...
Questions

Mathematics, 21.10.2020 23:01

Mathematics, 21.10.2020 23:01







Mathematics, 21.10.2020 23:01


Mathematics, 21.10.2020 23:01

Mathematics, 21.10.2020 23:01


Arts, 21.10.2020 23:01

History, 21.10.2020 23:01

English, 21.10.2020 23:01

Mathematics, 21.10.2020 23:01

Arts, 21.10.2020 23:01