
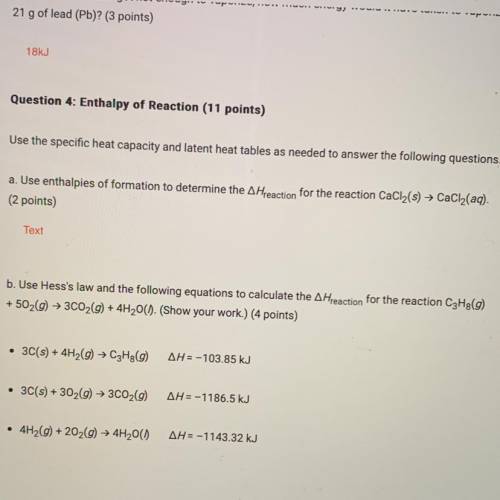
B. Use Hess's law and the following equations to calculate the A Hreaction for the reaction C3H8(9)
+ 502(9) + 3C02(9) + 4H2O(). (Show your work.) (4 points)
• 3C(s) + 4H2(9) → C3Hg(9)
AH = -103.85 kJ
• 3C(s) + 302(g) + 3C02(9)
AH=-1186.5 kJ
• 4H2(9) +202(9) + 4H2O()
AH=-1143.32 kJ

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 23.06.2019 02:50
For questions 1 and 2, consider the following experimental data.hydrogen emission lines were detected at the following wavelengths (in nm): 121.6102.697.395.093.8question 1use the electromagnetic radiation classifications below and figure 1-1 in the introductory information for this lab (in the lab manual) to determine the nf value for the experimental data provided? wavelength, ? (nm) 650 700 550 600 400 450 500 visible spectrum wavelength, ? (m) 11 10 3 10 10 10 8 10 5 10 10 -10 10 9 10 10 10 10 -12 10 microwave radio infrared x-ray ultraviolet gamma 1020 1019 1018 1 1016 015 1014 01 12 109108 frequency, v (hz)a.1b. 2c. 3d. 4e. 5question 2using the data for the emission line with the longest wavelength, the known value of nf (from question 1 in this prelab), and the value of ni (deduced from the ? and nf values) calculate the rydberg constant for hydrogen (rh) in units of m-1.a) 1.097 x 10-11 m-1b) 5.921 x 107 m-1c) 1.097 x 10-2 m-1d) 9.252 x 106 m-1e) 1.097 x 107 m-1
Answers: 3
You know the right answer?
B. Use Hess's law and the following equations to calculate the A Hreaction for the reaction C3H8(9)...
Questions

Mathematics, 26.01.2021 18:10


Business, 26.01.2021 18:10






English, 26.01.2021 18:10

Mathematics, 26.01.2021 18:10


English, 26.01.2021 18:10

Mathematics, 26.01.2021 18:10



Mathematics, 26.01.2021 18:10

Mathematics, 26.01.2021 18:10


Mathematics, 26.01.2021 18:10

Mathematics, 26.01.2021 18:10



