
Chemistry, 04.03.2021 07:10 sierram298
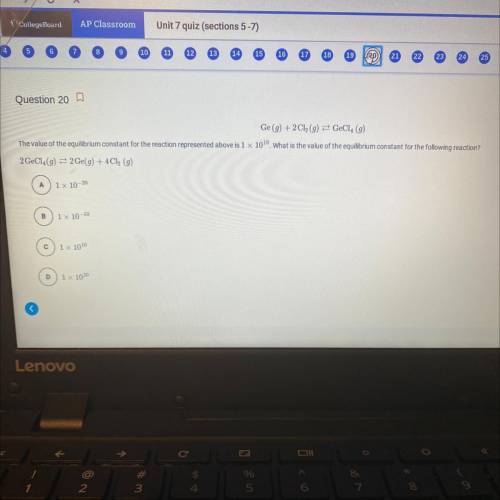
Ge (g) + 2Cl(g) = GeCl4 (g)
The value of the equilibrium constant for the reaction represented above is 1 x 100. What is the value of the equilibrium constant for the following reaction?
2 GeCl (g) = 2 Ge(g) +4Cl (g)
a) 1x 10-20
b) 1x 10-10
c) 1x 1010
d) 1 x 1020
will give brainliest !!

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
Ge (g) + 2Cl(g) = GeCl4 (g)
The value of the equilibrium constant for the reaction represented abov...
Questions







Mathematics, 05.02.2020 10:53

Chemistry, 05.02.2020 10:53







History, 05.02.2020 10:53



Biology, 05.02.2020 10:53




