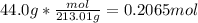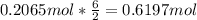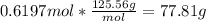6CuNO3 + Al2(SO4)3 → 3Cu2SO4 + 2Al(NO3)3
Molar mass of CuNO3 125.56 g/mol
Molar mass of Al(NO...

Chemistry, 04.03.2021 16:00 tasnimsas3
6CuNO3 + Al2(SO4)3 → 3Cu2SO4 + 2Al(NO3)3
Molar mass of CuNO3 125.56 g/mol
Molar mass of Al(NO3)3 213.01 g/mol
How many grams of copper (I) nitrate (CuNO3) are required to produce 44.0 grams of aluminum nitrate (Al(NO3)3)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1


Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
You know the right answer?
Questions









Mathematics, 23.12.2020 17:10


Mathematics, 23.12.2020 17:10


Social Studies, 23.12.2020 17:10


Mathematics, 23.12.2020 17:10

Mathematics, 23.12.2020 17:10

Mathematics, 23.12.2020 17:10



Mathematics, 23.12.2020 17:10