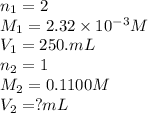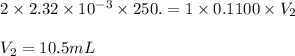Achemistry student weighs out 0.0475g of sulfurous acid h2so3, a diprotic acid, into a
250.ml...

Achemistry student weighs out 0.0475g of sulfurous acid h2so3, a diprotic acid, into a
250.ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1100m naoh solution.
calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
Questions

Biology, 11.02.2021 17:20


Mathematics, 11.02.2021 17:20




History, 11.02.2021 17:20




English, 11.02.2021 17:20

Mathematics, 11.02.2021 17:20





Computers and Technology, 11.02.2021 17:20


Chemistry, 11.02.2021 17:20

Mathematics, 11.02.2021 17:20

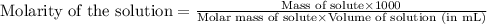
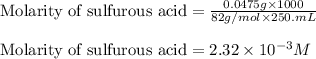
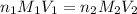
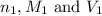 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 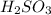
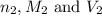 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.