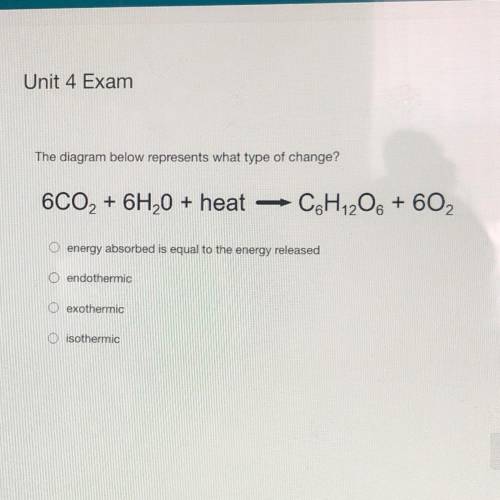
The diagram below represents what type of change?
6CO2 + 6H2O + heat
C&H 206 + 602
...

Chemistry, 04.03.2021 19:50 SoccerHalo
The diagram below represents what type of change?
6CO2 + 6H2O + heat
C&H 206 + 602
O energy absorbed is equal to the energy released
O endothermic
exothermic
O isothermic

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 22:30
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1

You know the right answer?
Questions




Arts, 21.11.2020 06:00

Social Studies, 21.11.2020 06:00

Mathematics, 21.11.2020 06:00

Mathematics, 21.11.2020 06:00

Mathematics, 21.11.2020 06:00

History, 21.11.2020 06:00

English, 21.11.2020 06:00




Mathematics, 21.11.2020 06:00

Business, 21.11.2020 06:00

Chemistry, 21.11.2020 06:00

Mathematics, 21.11.2020 06:00

Mathematics, 21.11.2020 06:00


History, 21.11.2020 06:00



