
Chemistry, 04.03.2021 21:00 brooket30057
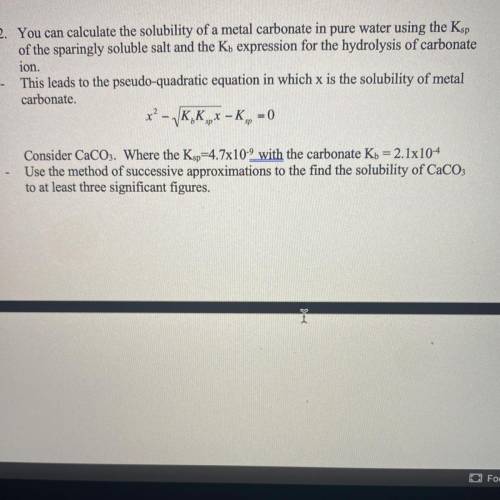
2. You can calculate the solubility of a metal carbonate in pure water using the Ksp
of the sparingly soluble salt and the Kb expression for the hydrolysis of carbonate
ion.
This leads to the pseudo-quadratic equation in which x is the solubility of metal
carbonate.
*? - VK, K,- K,, -
= 0
Consider CaCO3. Where the Ksp=4.7x10-9 with the carbonate Kb = 2.1x10-4
Use the method of successive approximations to the find the solubility of CaCO3
to at least three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

You know the right answer?
2. You can calculate the solubility of a metal carbonate in pure water using the Ksp
of the sparing...
Questions

Mathematics, 25.10.2020 01:00


English, 25.10.2020 01:00


Mathematics, 25.10.2020 01:00

Mathematics, 25.10.2020 01:00




Biology, 25.10.2020 01:00

Computers and Technology, 25.10.2020 01:00


Mathematics, 25.10.2020 01:00

Chemistry, 25.10.2020 01:00

Mathematics, 25.10.2020 01:00



Social Studies, 25.10.2020 01:00

English, 25.10.2020 01:00

Social Studies, 25.10.2020 01:00



