
Chemistry, 04.03.2021 21:40 kerstynsharp08
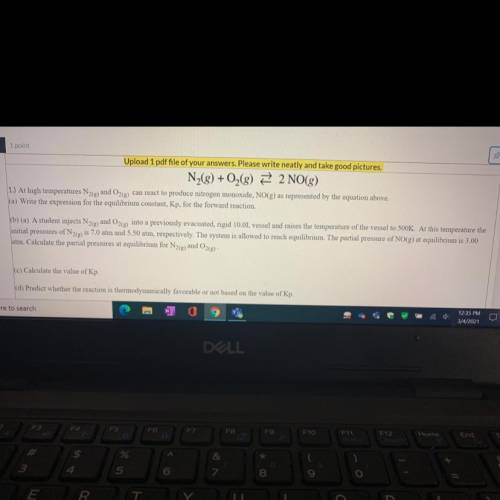
N2 + O2 —> 2NO
b) A student injects N2(g)and O2(g) into a previously evacuated, rigid 10.0L vessel and raises the temperature of the vessel to 500K. At this temperature the
initial pressures of N2(g) is 7.0 atm and 5.50 atm, respectively. The system is allowed to reach equilibrium. The partial pressure of NO(g) at equilibrium is 3.00
atm. Calculate the partial pressures at equilibrium for N2(e) and O2(g)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2


Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1
You know the right answer?
N2 + O2 —> 2NO
b) A student injects N2(g)and O2(g) into a previously evacuated, rigid 10.0L vess...
Questions



Mathematics, 23.02.2021 07:30

Geography, 23.02.2021 07:30

Mathematics, 23.02.2021 07:30

Biology, 23.02.2021 07:30

Mathematics, 23.02.2021 07:30

Spanish, 23.02.2021 07:30


Chemistry, 23.02.2021 07:30

Mathematics, 23.02.2021 07:30

Mathematics, 23.02.2021 07:30

Biology, 23.02.2021 07:30


Mathematics, 23.02.2021 07:30

Mathematics, 23.02.2021 07:30


Chemistry, 23.02.2021 07:30

English, 23.02.2021 07:30



